Few compounds in the field of pharmaceutical chemistry have had such a substantial impact on the treatment of illnesses connected to acidity as famotidine. Because famotidine is a strong histamine H₂-receptor antagonist, it reduces the formation of stomach acid, which helps those with GERD and peptic ulcers. Its careful organic chemistry-based synthesis and use as an active pharmaceutical ingredient (API) highlight the relationship between quality control, industrial scalability, and molecular design. From its chemical structure to its worldwide manufacture, the famotidine API molecule’s journey is explained in this article, along with the crucial problem of impurities that arise during synthesis.
1. The Molecular Blueprint: Decoding Famotidine’s Structure
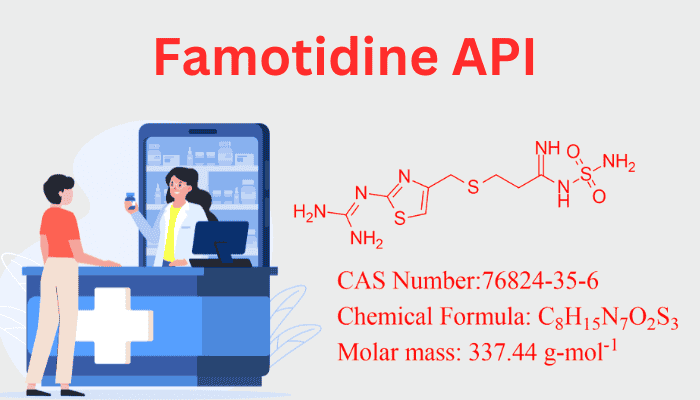
Famotidine (C₈H₁₅N₇O₂S₃) is a member of the thiazole class of chemicals and has a heterocyclic core that is rich in nitrogen and sulfur. Its structure includes:
- A thiazole ring linked to a guanidine group via a sulfonamide bridge.
- A diaminomethyleneamino side chain, which enhances receptor binding specificity.
Because of its special structure, famotidine can competitively inhibit histamine at gastric parietal cells’ H₂ receptors, lowering acid output by as much as 70%. Famotidine has a safer therapeutic profile because it doesn’t have antiandrogenic effects like older H₂ antagonists (like cimetidine).
2. Synthesis of Famotidine API: Precision in Multi-Step Chemistry
Famotidine API is made by a multi-step organic process that combines purification, cyclization, and nucleophilic substitution. This is a summary:
Step 1: Synthesis of the Thiazole Core
2-Chloroacetoacetate and thiourea react in an acidic media to create 2-aminothiazole-4-carboxylic acid ethyl ester at the start of the process. The thiazole ring is built on top of this intermediate.
Step 2: Introduction of the Guanidine Moiety
In the presence of a coupling agent (such as DCC), the ester is hydrolyzed to produce a carboxylic acid, which is subsequently treated with guanidine carbonate. The thiazole-guanidine backbone is formed in this phase.
Step 3: Sulfonamide Bridge Formation
The sulfonamide (-SO₂-NH-) bridge, which is essential for receptor engagement, is created when the guanidine derivative combines with 1,3-diaminopropane and sulfur trioxide.
Step 4: Final Functionalization
The final structure of famotidine is obtained by a Mannich reaction in which formaldehyde and dimethylamine react with the intermediate to add the diamino side chain.
Step 5: Purification
To reach >99.5% purity and adhere to pharmacopeial standards (USP, EP), crude famotidine is purified by crystallization (using ethanol/water combinations) and chromatography.
3. Sources of Famotidine API: Global Supply Chain Dynamics
A combination of innovative businesses and generic producers dominate the production of famotidine API:
- North America: generic companies such as Teva Pharmaceuticals and Merck & Co., the original patent holder.
- Asia: China (Zhejiang Huahai Pharmaceutical) and India (Sun Pharma, Aurobindo) are leaders in large-scale, reasonably priced synthesis.
- Europe: Sandoz (Novartis) and other companies prioritize high-purity APIs for regulated markets.
Regulatory-Oversight:
Batch consistency is ensured by strict adherence to GMP and FDA and EMA requirements. The FDA’s 2021 warning letter to a Chinese API manufacturer, for example, emphasized impurity control violations and emphasized the importance of quality.
4. Impurities in Famotidine API: Origins, Risks, and Control
Three main sources contribute to famotidine API impurities:
- Initial Materials: Unreacted intermediates or residual solvents, such as dimethylformamide.
- Degradation Products: When exposed to heat or moisture, the sulfonamide bridge hydrolyzes.
- Process-Related Byproducts: Synthesis-related side reactions, such as excessive alkylation.
Key Impurities Identified:
- Famotidine Sulfoxide: A result of oxidation that develops during storage.
- Desmethyl Famotidine: A byproduct of the Mannich process that undergoes demethylation.
- Thiazole Isomers: Structural variants with reduced efficacy.
Analytical-Control:
Impurity limitations are required by contemporary pharmacopeias (e.g., ≤0.15% for any unknown impurity). For detection, methods like NMR and HPLC-MS are used. For instance, a reverse-phase HPLC technique with UV detection at 254 nm is specified in the USP monograph for famotidine.
5. Challenges in Synthesis and Quality Assurance
- Complex Synthesis Pathway: Intermediate variability is more likely in multi-step processes.
- Thermal Sensitivity: Famotidine requires careful storage because it degrades at temperatures higher than 40°C.
- Environmental Impact: Waste from solvents, such DMF, needs to be recycled or replaced with more environmentally friendly options, like ionic liquids.
Case Study: Strong stability testing is necessary, as evidenced by a batch recall in Europe in 2020 that linked excessive sulfoxide levels to incorrect packaging.
6. Innovations and Future Directions
To enhance efficiency and sustainability, the industry is adopting:
- Flow Chemistry: Waste and reaction times are decreased by continuous synthesis.
- Biocatalysis: Enzymes that have been engineered to create bonds selectively while reducing byproducts.
- Advanced Purification: Membrane filtration and simulated moving bed (SMB) chromatography.
Co-crystal formulations are also being investigated by researchers to increase the solubility and shelf life of famotidine.
7. Conclusion: The Alchemy of Acid Suppression
The combination of molecular creativity and industrial rigor is embodied in famotidine API, from its globally lifesaving function to its meticulously engineered thiazole core. Its future will be determined by its emphasis on impurity control and green synthesis as demand rises due to an increase in the prevalence of GERD. For scientists, the tale of famotidine is proof that painstaking research may lead to valuable medicinal compounds.
“The most fruitful basis for the discovery of a new drug is to start with an old drug,” said pharmacologist Sir James Black, whose research on receptor antagonists led to the development of medications like famotidine. Building on its predecessors, famotidine keeps raising the bar for pharmaceutical excellence.
8. References (to be hyperlinked):
- USP-NF Monograph for Famotidine.
- FDA Guidance on Impurity Profiling (ICH Q3A/B).
- Journal of Pharmaceutical Sciences, “Advances in H₂ Antagonist Synthesis” (2022).
The goal of Molecular Chemistry is to shed light on the molecules influencing healthcare, and this investigation of famotidine’s chemistry fits that goal. For more information on the APIs that drive contemporary medicine, stay tuned!

Very useful thing sir thank you so much
This post is very helpful for science and pharmacy students 🤘