1. Introduction: Erythromycin’s Legacy in Antibiotic Therapy
A key component of macrolide antibiotics, erythromycin was discovered in 1952 from Saccharopolyspora erythraea and provides a vital substitute for people allergic to penicillins. Its position in contemporary medicine has been solidified by its molecular complexity and broad-spectrum effectiveness against Chlamydia, Mycoplasma, and Gram-positive bacteria. From microbial synthesis to quality control, this essay explains the chemistry and industrial complexities of erythromycin API, emphasizing its continued significance in an age of antibiotic resistance.
2. Molecular Architecture: The Macrolide Backbone
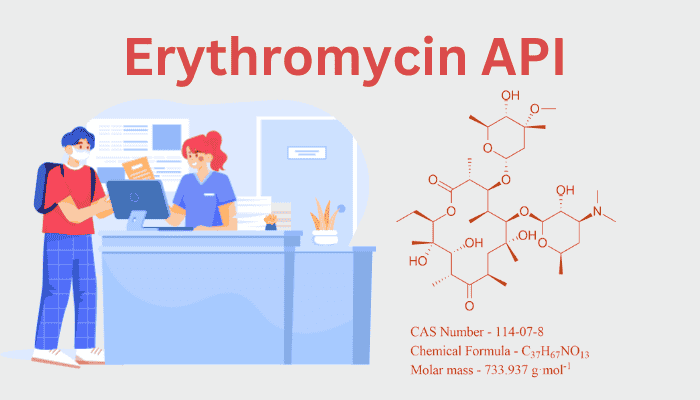
The 14-membered macrolide lactone ring of erythromycin (C₃₇H₆₇NO₁₃) is decorated with two deoxy sugars, cladinose and desosamine. Important structural components consist of:
- Hydroxyl and keto groups: Help bind to the 50S ribosomal subunit, which prevents the production of proteins by bacteria.
- Glycosidic linkages: Improve pharmacokinetics and solubility.
- Epimerization sensitivity: The structure of the C-12 hydroxyl group is essential for activity; its breakdown results in inert anhydroerythromycin.
Its therapeutic action and synthesis challenges are supported by this complex structure.
3. Synthesis of Erythromycin API: Fermentation and Precision
In S. erythraea, erythromycin is biosynthesised through polyketide synthase (PKS) pathways, a process that needs careful regulation:
Fermentation Process
- Strain Cultivation: Aerated bioreactors containing carbon sources (such as glucose) and nitrogen sources (soybean meal) are used to cultivate high-yield mutant strains.
- Precursor Feeding: Methylmalonyl-CoA and propionate lengthen the macrolide backbone by feeding the PKS machinery.
- Post-PKS Modifications: Cytochrome P450 enzymes and glycosyltransferases mediate the hydroxylation, glycosylation, and methylation processes that complete the structure.
Downstream Processing
- Harvesting: Organic solvents, such as butyl acetate, are used to extract erythromycin after mycelia are eliminated by filtering.
- Crystallization: Crude erythromycin is produced via pH-adjusted crystallization and then refined to >99% purity by chromatography.
4. Sources of Erythromycin API: Global Production Dynamics
Key Manufacturers:
- The original patent holder, Pfizer (USA), produces under the Ilosone name.
- Abbott Laboratories (USA): Provides pediatric suspensions of erythromycin ethylsuccinate.
- Leading manufacturers of generic APIs are North China Pharmaceutical Group (NCPC) and HEC Pharma (China).
Regulatory Compliance:
- Safety is ensured by following USP, EP, and ICH Q7 requirements.
- Microbial contamination and solvent residues are the main topics of FDA and EMA audits.
5. Formulation: Bridging API to Dosage Forms
Erythromycin’s instability in gastric acid and bitter taste drive innovative formulation strategies:
Dosage Forms
- Enteric-Coated Tablets: These shield the API (such as erythrocin) from stomach acid.
- Esters: Lactobionate (IV) and erythromycin ethylsuccinate (oral) increase solubility.
- Topical Ointments: Erygel and other acne treatments include 2% erythromycin.
Excipient Synergy
- Stearic acid: Enhances tablet compression.
- Hydroxypropyl methylcellulose (HPMC): Enteric coating polymer.
- Flavoring agents: Mask bitterness in suspensions.
6. Therapeutic Uses: From Clinics to Communities
- Respiratory Infections: Legionnaires’ disease, Pertussis.
- Dermatology: Acne vulgaris, erysipelas.
- STIs: Chlamydia, syphilis (penicillin-allergic patients).
- Prophylaxis: bacterial endocarditis, Rheumatic fever.
Dosage Regimens:
- Adults: 250–500 mg every 6 hours.
- Pediatrics: 30–50 mg/kg/day as ethylsuccinate.
7. Impurities: Challenges in Purity and Safety
Types of Impurities
- Related Substances: Erythromycin B and C (fermentation byproducts with reduced activity).
- Degradation Products: Anhydroerythromycin (acid-induced), erythromycin enol ether (heat-induced).
- Residual Solvents: Butyl acetate, methanol (limits per ICH Q3C).
- Heavy Metals: Lead, arsenic (<10 ppm as per USP).
Control Strategies
- HPLC-UV/HRMS: Quantifies impurities against reference standards.
- Stability Studies: Accelerated conditions (40°C/75% RH) predict degradation pathways.
- Crystallization Optimization: Reduces erythromycin B/C content to <0.5%.
8. Challenges and Innovations
Antibiotic Resistance:
- By methylating the ribosomal target, erm genes decrease the binding of erythromycin.
Green Chemistry Advances:
- Enzymatic Extraction: Lipases replace solvent-intensive steps.
- CRISPR-Engineered Strains: Boost yield by 30% in pilot studies.
Next-Gen Formulations:
- Nanoemulsions: Improve topical delivery for acne.
- Co-amorphous Systems: Enhance oral bioavailability with polymers.
9. Conclusion: Erythromycin’s Future in a Changing Landscape
Erythromycin’s chemical adaptability and formulation flexibility guarantee its stay in the antibiotic arsenal despite growing resistance. Biosynthesis and impurity control innovations are prime examples of how traditional molecules adapt to contemporary problems. “The antibiotic age is a perpetual race between ingenuity and oblivion,” as Nobel laureate Selman Waksman put it. Erythromycin keeps moving forward thanks to its complex chemistry and timeless usefulness.
10. References (to be hyperlinked):
- Journal of Industrial Microbiology & Biotechnology, “Advances in Erythromycin Biosynthesis” (2021).
- Pfizer’s Ilosone Product Insert.
- USP Monograph for Erythromycin.
- FDA Guidance on Macrolide Impurities (ICH Q3A).
This in-depth examination of the chemistry and industrial effects of erythromycin is consistent with Molecular Chemistry’s goal of unraveling the chemicals influencing healthcare. Watch this space for additional investigations on the science underlying life-saving APIs!