1. Introduction: Celecoxib’s Role in Modern Pain Management
When it was first introduced in 1999, celecoxib, a groundbreaking selective cyclooxygenase-2 (COX-2) inhibitor, completely changed how pain and inflammation were treated. Being the first FDA-approved COX-2 inhibitor, it reduced gastrointestinal adverse effects and provided a safer substitute for conventional NSAIDs. In addition to discussing the crucial difficulties of impurity control in its manufacturing, this article explores the chemistry, industrial synthesis, and medicinal uses of celecoxib API.
2. Molecular Architecture: The Design of a Selective COX-2 Inhibitor
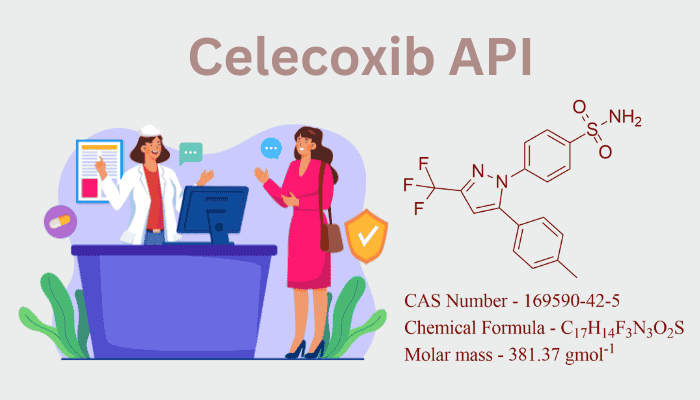
For COX-2 selectivity, celecoxib (C₁₇H₁₄F₃N₃O₂S) has a bicyclic sulfonamide structure. Important components consist of:
- A central pyrazole ring: Serves as the scaffold for substituents.
- Trifluoromethyl (-CF₃) and sulfonamide (-SO₂NH₂) groups: Critical for binding to the COX-2 enzyme’s hydrophobic pocket.
- Para-methylphenyl group: Enhances pharmacokinetic stability.
Because of its nature, celecoxib can block the pro-inflammatory enzyme COX-2 while protecting the gastric mucosa with COX-1, lowering the incidence of ulcers by about 50% when compared to non-selective NSAIDs.
3. Synthesis of Celecoxib API: Precision in Heterocyclic Chemistry
The synthesis of celecoxib involves several steps that combine cyclization and nucleophilic substitution:
Step 1: Synthesis of 1,3-Diketone Intermediate
- Reaction: In the presence of sodium hydride, 4-methylacetophenone and ethyl trifluoroacetate condense to create 1-(4-methylphenyl)-4,4,4-trifluorobutane-1,3-dione.
Step 2: Formation of the Pyrazole Core
- Cyclization: The pyrazole ring is closed by the reaction between the diketone and hydrazine hydrate, resulting in 4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyrazol-1-yl]benzenesulfonamide.
Step 3: Sulfonamide Functionalization
- Sulfonation: The sulfonamide group is added to the intermediate by ammonolysis after it has been treated with chlorosulfonic acid.
Step 4: Purification
- Crystallization: Methanol/water mixtures refine the crude product.
- Chromatography: Silica gel chromatography removes residual hydrazine and solvents.
Final API purity exceeds 99.5%, complying with USP/EP standards.
4. Sources of Celecoxib API: Global Production Landscape
Key Manufacturers:
- Pfizer (USA): Original patent holder, markets branded celecoxib as Celebrex.
- Teva Pharmaceuticals (Israel): Leading generic supplier.
- Dr. Reddy’s (India) and Zhejiang Huahai (China): Bulk API producers for emerging markets.
Regulatory Compliance:
- FDA/EMA Oversight: thorough examinations for genotoxic contaminants, such as hydrazine residues.
- Patents: Key patents expired in 2014, enabling generic competition.
5. Formulation: From API to Patient-Friendly Dosage
Celecoxib’s low solubility (7 µg/mL) drives formulation innovation:
Dosage Forms:
- Capsules (50–400 mg): Contain microcrystalline cellulose and lactose.
- Oral Suspension: Uses polysorbate 80 to enhance solubility.
Excipient Synergy:
- Sodium lauryl sulfate: Wetting agent to improve dissolution.
- Titanium dioxide: Opacifier in capsules.
- Gelatin: Capsule shell material.
Stability Considerations:
- Packaging: Desiccant-containing bottles prevent moisture-induced degradation.
- Storage: Recommended below 25°C.
6. Target Uses: Therapeutic Applications and Dosage
Celecoxib’s COX-2 selectivity makes it ideal for:
- Osteoarthritis and Rheumatoid Arthritis: lowers joint pain and inflammation (100–200 mg twice a day).
- Acute Pain: Postoperative dental pain (400 mg initial dose).
- Familial Adenomatous Polyposis (FAP): 400 mg twice a day (off-label) to decrease colorectal polyps.
Contraindications:
- Steered clear of in patients with cardiovascular disease because of the danger of thromboembolism (associated with COX-2 inhibition).
7. Impurities: Origins, Risks, and Analytical Control
Critical Impurities:
- Hydrazine: Genotoxic residue from pyrazole cyclization (limit: <1 ppm per ICH M7).
- Sulfonic Acid Derivatives: Over-sulfonation byproducts.
- Des-methyl Celecoxib: Incomplete methylation during synthesis.
Control Strategies:
- HPLC-MS: Quantifies impurities against reference standards.
- QbD (Quality by Design): Optimizes reaction conditions to minimize hydrazine formation.
8. Challenges and Innovations
Safety Concerns:
- Cardiovascular Risks: The FDA requires black-box warnings for all COX-2 medicines following the Vioxx incident.
Green Chemistry Advances:
- Continuous Flow Synthesis: Reduces solvent use by 40% in pilot studies.
- Enzymatic Sulfonation: Sulfotransferases are investigated for environmentally friendly functionalization.
Next-Gen Formulations:
- Nanoemulsions: Enhance bioavailability for pediatric use.
- Transdermal Patches: Bypass first-pass metabolism.
9. Conclusion: Celecoxib’s Legacy and Future
Celecoxib is still an example of tailored molecular design that manages inflammation while striking a balance between safety and effectiveness. Its use may spread into neurology and oncology as green synthesis and customized medicine research progress. John Vane, a pharmacologist and Nobel winner for his work on COX, once said: “Knowing enzymes is knowing life.” This fact is demonstrated by the tale of celecoxib, which demonstrates that chemistry is the foundation of innovative therapeutics.
10. References (to be hyperlinked):
- USP Monograph for Celecoxib.
- Pfizer’s Celebrex Product Insert.
- FDA Guidance on COX-2 Inhibitors (2023).
- Journal of Medicinal Chemistry, “Advances in Pyrazole Synthesis” (2021).
Molecular Chemistry’s goal of deciphering the molecules influencing healthcare is in line with this investigation of celecoxib’s chemistry and industrial effects. Watch this space for additional insights into the science underlying innovative medications!