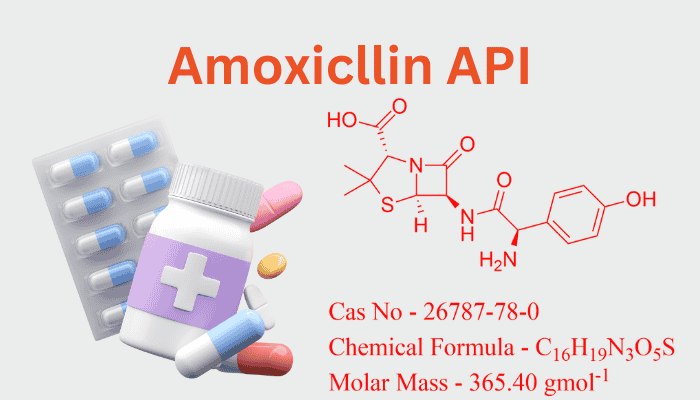
Amoxicillin is one of the few compounds in the broad field of pharmaceutical chemistry that has had such a significant influence on world health. The synthesis and manufacture of amoxicillin, a key member of the β-lactam antibiotic family, is a triumph of industrial innovation, microbial fermentation, and organic chemistry. This article explores the scientific and industrial frameworks that support the manufacture of amoxicillin, delving into its complex journey from its molecular blueprint to its role in fighting bacterial infections.
1. The Molecular Backbone: Understanding Amoxicillin’s Structure
A key component of amoxicillin’s bactericidal action is its β-lactam ring linked to a thiazolidine ring, which makes it a member of the aminopenicillin subclass (C₁₆H₁₉N₃O₅S). Amoxicillin differs from penicillin in that it has a hydroxyl group (-OH) and an extra amino group (-NH₂) on its benzene side chain (see Figure 1). These changes improve its:
- Acid stability: Survives stomach acid for oral bioavailability.
- Broad-spectrum activity: Targets Gram-positive and some Gram-negative bacteria.
- Resistance to β-lactamase enzymes: Though later formulations often pair it with clavulanic acid for added protection.
This molecular architecture not only dictates its therapeutic efficacy but also guides the synthetic pathways used to produce it at scale.
2. Synthesis of Amoxicillin API: A Fusion of Fermentation and Chemistry
The production of amoxicillin API is a semi-synthetic process, blending microbial fermentation with chemical modification. Here’s a step-by-step breakdown:
Step 1: Fermentation of Penicillin G
The voyage begins with the fermentation of Penicillium chrysogenum, a mold cultivated in enormous bioreactors. The crucial β-lactam molecule, penicillin G (benzylpenicillin), is secreted by the fungus under regulated pH, temperature, and aeration conditions.
Step 2: Isolation and Hydrolysis
Using immobilized penicillin acylase, penicillin G is separated from the fermentation broth and then hydrolyzed enzymatically. The primary scaffold for all semi-synthetic penicillins, 6-aminopenicillanic acid (6-APA), is produced when this enzyme breaks down the side chain of penicillin G.
Step 3: Chemical Acylation
6-APA is nucleophilically acylated with a side chain precursor, D-(-)-p-hydroxyphenylglycine (HPG). Amoxicillin is created by this reaction, which is usually aided by a coupling agent such as DCC (dicyclohexylcarbodiimide), which adds the special hydroxyl and amino groups.
Step 4: Purification and Crystallization
Using chromatography and crystallization, the crude product is refined to satisfy pharmacopeial requirements (e.g., USP, EP). To guarantee >99% purity, impurities like as unreacted intermediates or leftover solvents are carefully eliminated.
3. Sources of Amoxicillin API: Global Production Landscape
Amoxicillin API is manufactured by a mix of multinational pharmaceutical giants and specialized API producers, primarily concentrated in:
Asia: Using affordable fermentation infrastructure, China and India control the world’s supply.
Europe: Businesses that prioritize high-quality, sustainable production include Sandoz (Switzerland) and DSM (Netherlands).
North America: Pfizer and Aurobindo Pharma continue to have large regional distribution capacity.
Key Players:
- Dr. Reddy’s Laboratories (India)
- United Laboratories (China)
- ACS Dobfar (Italy)
Regulatory oversight (e.g., FDA, EMA) ensures adherence to Good Manufacturing Practices (GMP), critical for maintaining efficacy and safety.
4. Industrial Applications: From API to Tablet
Once synthesized, amoxicillin API is formulated into diverse dosage forms:
- Oral tablets/capsules: Most common, often combined with clavulanic acid (e.g., Augmentin).
- Pediatric suspensions: Water-soluble powders for easier dosing.
- Injectable solutions: For severe infections in hospital settings.
Market-Impact:
According to Grand View Research, the API market for amoxicillin was estimated to be worth $4.2 billion in 2023, making it the most prescribed antibiotic in the world. It is essential for treating urinary tract infections, strep throat, and pneumonia due to its effectiveness and affordability.
5. Challenges in Synthesis and Production
Despite its ubiquity, amoxicillin production faces hurdles:
• Scarcity of raw materials: Supplies of HPG and 6-APA are susceptible to environmental and geopolitical upheavals.
• Environmental impact: Businesses are using green chemistry techniques (e.g., solvent recycling, enzymatic catalysis) in response to the substantial waste generated by fermentation.
• Antimicrobial resistance (AMR): The need for new β-lactamase inhibitors is being driven by resistant organisms that have been induced by overuse.
6. Innovations and Future Directions
To address these challenges, the industry is pivoting toward:
- Continuous manufacturing: Replacing batch processes for higher efficiency.
- Biocatalysis: Engineered enzymes for greener, more selective synthesis.
- Polymer-supported reagents: Reducing purification steps and waste.
Researchers are also exploring prodrug formulations and nanoparticle delivery systems to enhance amoxicillin’s bioavailability and target specificity.
7. Conclusion: The Alchemy of Saving Lives
The history of amoxicillin API demonstrates how organic chemistry and industrial pragmatism can work together harmoniously. Every gram of amoxicillin represents decades of scientific ingenuity and rigor, from the fungal vats of Penicillium to the accuracy of contemporary synthesis. The development of the molecule, driven by advanced technology and green chemistry, will continue to be essential in preserving world health as bacterial resistance approaches.
The synthesis of amoxicillin is more than just a procedure to chemists; it serves as a reminder of how molecular creativity may lead to life-saving uses. “One sometimes finds what one is not looking for,” said Alexander Fleming, whose discovery of penicillin served as the impetus for this adventure. But in the case of amoxicillin, the world discovered just what it required.

Keep it up guru ji 🙌