1. Introduction: Clarithromycin’s Role in Modern Therapeutics
Since receiving FDA clearance in 1991, the semi-synthetic macrolide antibiotic clarithromycin has been a mainstay in the treatment of bacterial infections. Derived from erythromycin, it is a recommended treatment for gastrointestinal, dermatological, and respiratory infections due to its improved pharmacokinetic profile and acid stability, which address significant shortcomings of its predecessor. The chemistry, industrial synthesis, and medicinal uses of clarithromycin API are examined in this article, along with the difficulties in controlling impurities during manufacture.
2. Molecular Architecture: The Evolution from Erythromycin
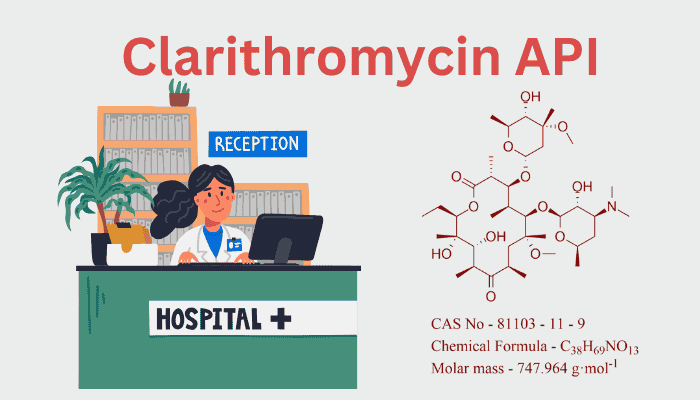
The 14-membered macrolide lactone ring of clarithromycin (C₃₈H₆₉NO₁₃) is similar to that of erythromycin, except it has a 6-O-methyl group, a change that:
- Enhances acid stability: prevents breakdown in stomach acid, allowing for enteric coating-free oral delivery.
- Improves bioavailability: Greater tissue penetration and a longer half-life (around five hours as opposed to 1.5 hours for erythromycin).
- Reduces gastrointestinal side effects: reduces the nausea-causing motilin receptor agonism caused by erythromycin.
The molecule retains two deoxy sugars—desosamine and cladinose—critical for binding the bacterial 50S ribosomal subunit and inhibiting protein synthesis.
3. Synthesis of Clarithromycin API: Bridging Fermentation and Chemistry
The semi-synthetic method of producing clarithromycin combines precise chemical modification with microbial biosynthesis:
Step 1: Fermentation of Erythromycin
- Microbial Strain: Saccharopolyspora erythraea is cultured in aerated bioreactors with carbon (glucose) and nitrogen (soybean meal) sources.
- Polyketide Synthesis: Propionyl-CoA and methylmalonyl-CoA precursors are used by the organism’s PKS enzymes to construct the macrolide backbone.
- Post-PKS Modifications: Desosamine and cladinose are added by hydroxylation and glycosylation processes.
Step 2: Chemical Methylation
- Reaction: In the presence of a base (such as potassium carbonate), erythromycin’s C-6 hydroxyl group is O-methylated using methyl chloride or dimethyl sulfate.
- Solvent System: To increase reactivity, experiments are carried out in polar aprotic solvents such as dimethylformamide (DMF).
- Purification: Solvent extraction is used to separate the crude product, which is then crystallized to a purity of >99%.
Step 3: Final Processing
- Crystallization: High-purity clarithromycin crystals are produced from ethanol/water combinations.
- Drying: The API is made ready for formulation by lyophilization or spray drying.
4. Sources of Clarithromycin API: Global Production and Key Players
Major Manufacturers:
- AbbVie (USA): Markets branded clarithromycin as Biaxin.
- Teva Pharmaceuticals (Israel): Leading generic supplier.
- Lupin Ltd. (India) and Hisun Pharma (China): Dominate cost-effective bulk production.
Regulatory Landscape:
- FDA/EMA Compliance: Adherence to ICH Q7 (GMP) and ICH Q3A (impurity limits).
- Patents: The early 2000s saw the expiration of important patents, opening the door for the mass production of generics.
5. Formulation: From API to Patient-Ready Dosage
Clarithromycin’s stability and solubility dictate its formulation strategies:
Dosage Forms:
- Immediate-Release Tablets (250–500 mg): These include croscarmellose sodium (disintegrant) and microcrystalline cellulose (binder).
- Extended-Release Tablets: For regulated release, use hydroxypropyl methylcellulose (HPMC).
- Oral Suspension: Reconstituted powder with tastes (like cherry) to cover off bitterness.
Excipient Synergy:
- Magnesium stearate: Lubricant for tablet compression.
- Lactose: Filler enhancing flow properties.
- Citric acid: pH adjuster in suspensions.
Stability Considerations:
- Packaging: Blister packs protect tablets from moisture.
- Storage: Recommended at 15–30°C to prevent degradation.
6. Target Uses: Therapeutic Applications and Dosage
Clarithromycin’s broad-spectrum activity targets:
- Gram-positive bacteria: Streptococcus pneumoniae, Staphylococcus aureus.
- Atypical pathogens: Mycoplasma pneumoniae, Legionella pneumophila.
- Acid-fast bacteria: Mycobacterium avium complex (MAC).
Key Indications:
- Respiratory Infections: Community-acquired pneumonia, acute bronchitis.
- Skin/Soft Tissue Infections: Cellulitis, erysipelas.
- Helicobacter pylori Eradication: Triple therapy with amoxicillin and a PPI.
- MAC Prophylaxis: In HIV/AIDS patients.
Dosage Regimens:
- Adults: 250–500 mg twice daily.
- Pediatrics: 7.5 mg/kg twice daily (oral suspension).
7. Impurities: Origins, Risks, and Control
Types of Impurities:
- Related Substances:
- Erythromycin derivatives: Residual unmethylated intermediates.
- 6,11-Di-O-methylerythromycin: Over-methylation byproduct.
- Degradation Products:
- Clarithromycin sulfoxide: Oxidation under light/heat.
- N-Demethylclarithromycin: Hydrolytic degradation.
- Residual Solvents: DMF, methanol (ICH Q3C limits: <880 ppm for DMF).
Analytical Control:
- HPLC-UV/LC-MS: Quantifies impurities against USP/EP reference standards.
- Forced Degradation Studies: Expose API to heat, light, and humidity to identify degradation pathways.
8. Challenges and Innovations
Antibiotic Resistance:
- By methylating the 23S rRNA target, erm genes decrease the binding of clarithromycin.
- Combination Therapies: Using metronidazole or amoxicillin together reduces resistance to H. pylori treatment.
Green Chemistry Advances:
- Enzymatic Methylation: Engineered methyltransferases reduce solvent use.
- Continuous Flow Reactors: In the methylation processes, increase yield and decrease waste.
Next-Gen Formulations:
- Nanoencapsulation: Improves bioavailability in immunocompromised patients.
- Co-amorphous Systems: Combine clarithromycin with polymers for stability.
9. Conclusion: Clarithromycin’s Enduring Legacy
Clarithromycin is a prime example of how molecular modifications can transform medication effectiveness and patient adherence. Its use in combination treatments and continuous formulation advancements guarantee its continued importance as antibiotic resistance approaches. “The magic bullet is not a myth,” as pharmacologist Paul Ehrlich once said. Clarithromycin continues to be effective because to its accuracy and versatility.
10. References (to be hyperlinked):
- FDA Guidance on Macrolide Antibiotics (2023).
- USP Monograph for Clarithromycin.
- AbbVie’s Biaxin Product Insert.
- Journal of Antimicrobial Chemotherapy, “Clarithromycin in H. pylori Eradication” (2022).
This thorough examination of the chemistry and industrial effects of clarithromycin is in line with Molecular Chemistry’s goal of deciphering the chemicals influencing world health. Watch this space for additional insights into the science underlying medications that can save lives!

Keep it up guru ji 🙌
This is very helpful topic for science and pharmacy students